Exhibition
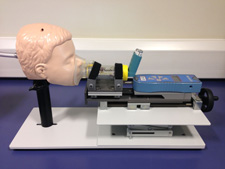
Copley Scientific is exhibiting its new face mask testing apparatus
During coffee breaks, lunch, and the drinks reception sponsored by Vectura at the end of the day, attendees had the opportunity to visit with exhibitors. Few companies seemed to be introducing new products or services, although there were some exceptions.
Among the new products on display was Copley Scientific’s face mask testing apparatus, which is designed to meet the requirements of draft USP chapter <1602> for testing spacers and valved holding chambers. The apparatus includes adjustable face models, replaceable skins, and a mechanism for controlling the force with which the facemask is applied to the model.

Cascade Technologies’ MDI canister leak detection system
Cascade Technologies debuted its MDI canister leak detection system, with a laser-based technology that detects propellant in the air surrounding the can at up to 200 cans per minute. DDL 25 was the first OINDP meeting for the Scottish company, which makes laser gas emission monitors and analyzers for a wide range of industries.
Iconovo’s DPI range, including the new ICOres inhaler
Swedish device company Iconovo showed off its new ICOres reservoir-based dry powder inhaler, part of a range of DPIs that include a capsule-based device, a pre-metered device, and a disposable unit dose device. According to the company, the ICOres can be specifically designed for bioequivalence with a Turbuhaler.
At Presspart’s booth, attendees practiced their golf shots at a simulator, and the company was promoting its new Quantum end of life dose indicator, which is built into the MDI canister and has no mechanical parts.
A few companies were promoting new services, including Skyepharma, which has added an MG2 Planeta capsule filling machine as part of its expanded DPI product development service, and Catalent, which recently acquired Micron Technologies, a micronization and powder characterization company.


