Selecting an appropriate nebulizer for the application
It is oftentimes helpful to compare and contrast the wide variety of nebulizer types across the entire spectrum of devices, based on one or more key attributes of interest:
Medication delivery
Common concerns related to delivery of the medication by a nebulizer include whether or not the delivery is assured, the level of fugitive emissions, the amount of time necessary to deliver the medication, and the fill volume or the dead volume, which is the amount of liquid containing medication that is trapped inside the nebulizer and is thus not made available for inhalation. The table below summarizes these attributes for each of the nebulizer types:
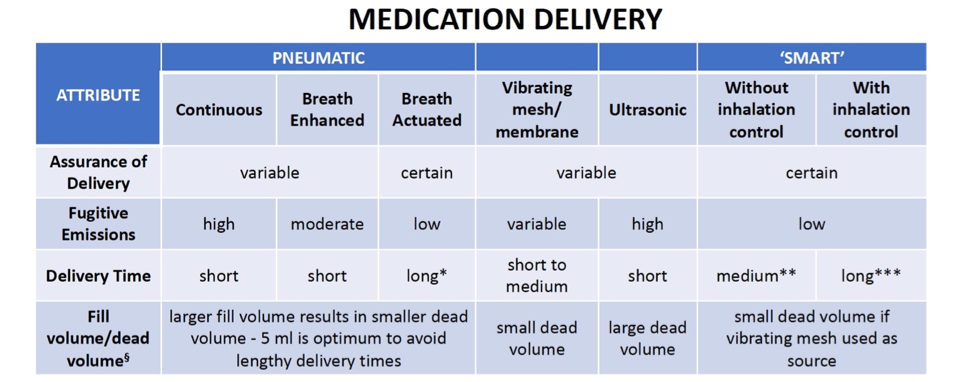
Note that while a shorter delivery time may be desirable in most cases, nebulizers with breath control may offer better delivery over a longer amount of time. For example, breath actuated pneumatic nebulizers take longer to deliver the medication than continuous pneumatic nebulizers because the medication is conserved rather than wasted during the time that the patient exhales.
Longer delivery times may also occur due to certain features of smart nebulizers that are designed to insure proper delivery; for example, some arrangements deliberately require the patient to inhale very slowly for a long period of time to improve deep lung penetration by the fine droplets. Delivery time may also be lengthened if the patient fails to meet the inhalation profile acceptance limits set for the nebulizer or if the patient cannot breathe comfortably with the breath control feature. Also, unless the nebulizer is breath actuated, delivery of all the medication cannot be assured because the device will deliver medication even if the patient removes the mouthpiece or facemask.
Formulation compatibility
Selection of a nebulizer type may be constrained by the nature of the formulation to be delivered. For example, while most types of nebulizer can deliver either suspensions or solutions, ultrasonic nebulizers cannot deliver suspensions, and vibrating mesh/membrane nebulizers cannot deliver suspensions efficiently where the particle size is close to or exceeds the nominal mesh nozzle diameter.
Efficient delivery of large molecule formulations may also be limited due to shear forces generated by pneumatic nebulizers or impossible due to the heating that occurs in ultrasonic nebulizers.
Manufacturability and use
Obviously, the more complex the manufacture of the nebulizer is, the more expensive it is likely to be, especially when the device requires a lot of electronics, connectivity, etc. Pneumatic nebulizers without any breath control are the simplest type available, which contributes to their low cost. Pneumatic nebulizers are also easy for patients or caregivers to use, though they may require set-up of a portable compressor for home use, and cannot be customized.


