 A new animation from Copley Scientific shows how the standard compendial set-up for cascade impaction can be enhanced to ensure that data generated in the lab (in vitro) is as representative as possible of what happens in clinic (in vivo).
A new animation from Copley Scientific shows how the standard compendial set-up for cascade impaction can be enhanced to ensure that data generated in the lab (in vitro) is as representative as possible of what happens in clinic (in vivo).
Demonstrating in vitro/in vivo correlation (IVIVC) is an important goal for generic developers, for the robust demonstration of bioequivalence, and, more generally, for the further advancement of inhalation technologies.
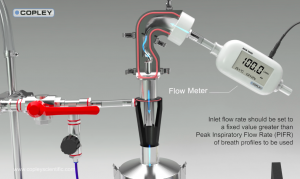
“System for improved in vitro-in vivo correlations (IVIVCs) of inhaled drug products,” shows a cascade impactor set-up that includes the Alberta Idealized Throat (AIT), a mixing inlet, and a breathing simulator.
Viewers get a look at flow through the human-like internal geometry of the AIT, an alternative to the standard USP induction port (IP), that has demonstrated the ability to deliver data that reflects observed deposition in the throat more closely that the standard USP IP.
With a breathing simulator, product performance can be evaluated using a more realistic inhalation profile than the on/off air flow used in standard inhaler testing methods, and a mixing inlet decouples the flow rate through the impactor from the flow rate through the inhaler, enabling the impactor to operate as a constant flow-rate aerosol sampler while at the same time permitting the application of a representative inhalation profile through the inhaler.
The animation demonstrates how to generate steady-state flow through the impactor using a compressed air source and the mixing inlet, prior to an accurate breath profile being generated by the breath simulator at the inlet. The video then shows particle entrainment as particles move through from the inhaler on their route to the impactor.
View the animation below:
Note: Newcomers to cascade impaction may wish to view this animation of the basic set-up first.
https://vimeo.com/178588373


