Following a short break for refreshments and poster viewing, the first session, titled “Drug Development – New Drugs, Targets and Formulations” got underway. The five papers presented in the session described the development of OINDPs targeting the heart and the brain, for indications including pulmonary arterial hypertension, migraine, and Alzheimer’s disease, plus the creation of a molecule combining a muscarinic antagonist and a beta agonist to create a “superbronchodilator.”
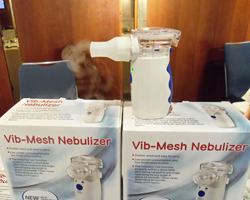
Health & Life’s new nebulizer
More than 50 exhibitors are taking part in the technology exhibition, with a number of companies participating for the first time. One of those, Health & Life from Taiwan, was demonstrating a new portable nebulizer that the company says can operate at any angle, including upside down, for up to 10 seconds and which can deliver 1 ml of inhalation solution in as little as 20 seconds.
The Health & Life nebulizer was one of few product launches at the exhibition; many of the returning exhibitors had other priorities. Team Consulting was handing out a new booklet on human factors engineering, while CRO Pharmasol & Pharmaserve NW was recruiting for a number of positions, as was Catalent.
After lunch, attendees had the opportunity to attend three of twelve hour-long workshops offered by suppliers. Workshops by DPT on “Formulation and Device – Critical Parameters for Development of OINDPs” and by Charles River Laboratories on “Preclinical Development for Inhalation Drugs” appeared to be particularly popular.
In the evening, the AAPS Inhalation and Nasal Technology Focus Group (INTFG) meeting featured a presentation by Günther Hochhaus of the University of Florida titled, “Pulmonary Equivalence: Is there any news?” INTFG organizers also wanted to use the meeting to promote their upcoming workshop at the USP this fall on “Inhaled Drug Products: Current Practices and the Future of In Vitro Testing Technologies and Regulation.”


